Eleventh paper from a Short-Term Scientific Mission (STSM) (September 6, 2016)
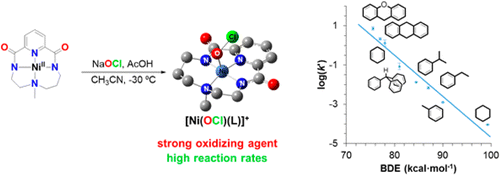
In this work, the group of Dr. Anna Company reports the catalytic activity of a nickel(II) complex in the chlorination and oxidation of C-H bonds using sodium hypochlorite as oxidant in the presence of acetic acid. Insight into the active species responsible for the observed chemistry was gained through the study of the reaction of the nickel(II) complex with hypochlorite at low temperature by UV-vis absorption, resonance Raman, EPR, ESI-MS and XAS analyses. DFT calculations aided the assignment of the trapped chromophoric species as a nickel-hypochlorite species. Despite the fact that the formal oxidation state of the nickel is +4, experimental and computational analysis indicate that this species is best formulated as a nickel(III) complex with one unpaired electron delocalized in the ligands surrounding the metal center. Most remarkable, this compound rapidly reacts with a range of substrates including those with strong aliphatic C-H bonds, indicating that this species is directly involved in the oxidation/chlorination reactions observed in the catalytic experiments.
EPR and resonance Raman experiments were performed in the group of Prof. Wesley Browne in the framework of a STSM by Teresa Corona who is a PhD student of Anna Company (ECOST-STSM-CM1305-32120). This work was recently published in J. Am. Chem. Soc.